When a gas is heated to a very high temperature or exposed to an intense electric field, it can become ionized and transform into a plasma. A plasma is an ionized gas consisting of charged particles, such as ions and electrons, as well as neutral particles. In a plasma, the molecules of the gas are broken down into their constituent ions and electrons, which interact with each other in a variety of ways.
The transition from a gas to a plasma involves several distinct steps, which we will explore in more detail below.
Step 1: Ionization The first step in the formation of a plasma is ionization. Ionization occurs when an electron is stripped from an atom or molecule, leaving behind a positively charged ion and a free electron. This process can occur through a variety of mechanisms, such as collisional ionization, photoionization, or electron impact ionization.
Collisional ionization occurs when a high-energy particle, such as an electron or ion, collides with an atom or molecule and transfers enough energy to remove one or more electrons. Photoionization occurs when a photon of light with enough energy interacts with an atom or molecule and knocks an electron off. Electron impact ionization occurs when a free electron collides with an atom or molecule and transfers enough energy to knock an electron off.
Step 2: Recombination After ionization, the plasma contains both positively charged ions and free electrons. These charged particles can interact with each other in a variety of ways, including recombination. Recombination occurs when a free electron is captured by a positively charged ion, neutralizing both particles and forming a new, neutral atom or molecule.
Step 3: Excitation and de-excitation In addition to ionization and recombination, plasma particles can also become excited. Excitation occurs when a plasma particle absorbs energy, causing its electrons to jump to higher energy levels. De-excitation occurs when an excited particle releases its excess energy, causing its electrons to drop back down to lower energy levels.
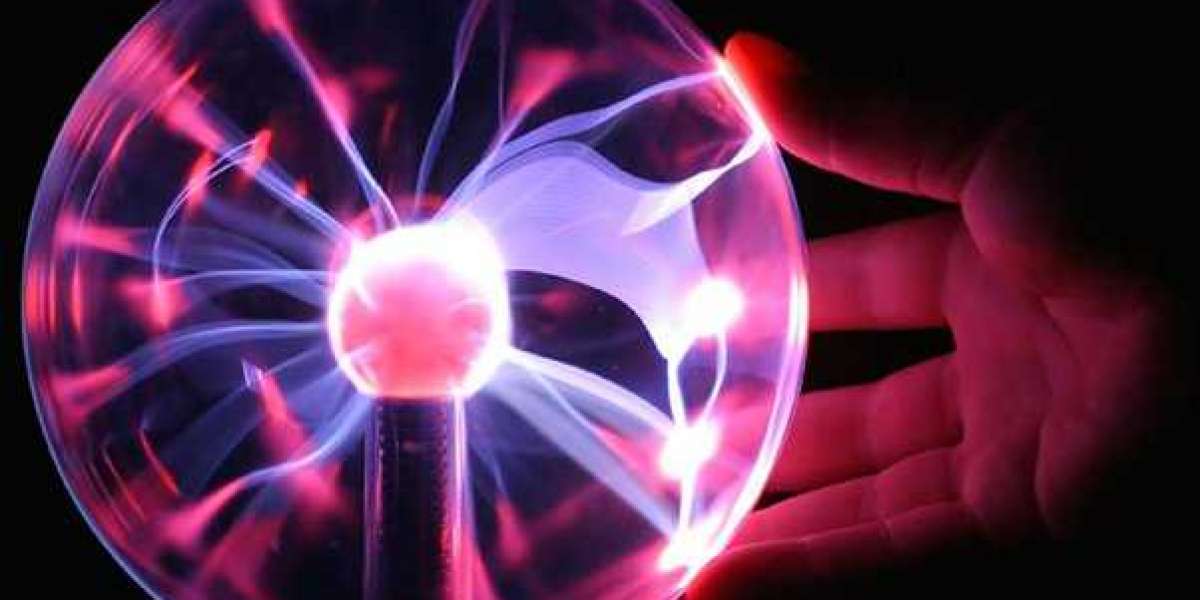
Excitation and de-excitation are important processes in plasma physics, as they can lead to the emission and absorption of light. When an excited plasma particle de-excites, it releases energy in the form of a photon of light. This process is responsible for the characteristic colors of many types of plasmas, such as the pink glow of a neon sign or the blue-green light of a plasma ball.
Step 4: Plasma behavior As a plasma forms and its particles interact with each other, a number of interesting and complex behaviors can emerge. For example, plasmas can exhibit collective phenomena such as plasma waves, which are oscillations of the plasma particles that can propagate through the plasma. These waves can be used in a variety of applications, such as plasma processing or plasma-based communication systems.
Plasmas can also exhibit magnetic behavior, as the motion of the charged particles can generate magnetic fields. This can lead to phenomena such as magnetic confinement, which is used in fusion experiments to contain high-temperature plasmas and achieve fusion reactions.
Overall, the transformation of a gas into a plasma involves the ionization of gas molecules, the subsequent recombination and excitation of plasma particles, and the emergence of complex behaviors as the plasma particles interact with each other. While plasmas can be challenging to understand and manipulate, they are also incredibly useful and have a wide range of applications in fields such as materials processing, energy production, and space exploration.



Mimi 4 w
Thank you